Minerals
Properties of Silver
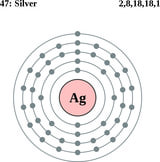
Silver is a bright white and lustrous metallic element that can conduct electricity and heat better than any other known metal. Silver is one of a number of transition metal in the periodic table of elements and has an atomic number of 47. Since the ancient times, the metal has long been known and valued a coinage and ornamental element.

Silver mines that are found in Asia Minor were possibly used before 2500 B.C. The alchemists commonly referred to the metal as Diana or Luna after the goddess of the moon and assigned the symbol of a crescent moon to the metal. The element’s name was derived from the Anglo-Saxon term for the metal. With the exemption of gold, silver is considered as the most ductile and malleable of all the known metals. Its hardness ranges 2.5 to 2.7 out of 10.
Silver is softer than copper but harder than gold. Silver has a melting point of around 1764°F or 962°C, boiling point of around 3924°F or 2162°C, a specific gravity of around 10.5, and an atomic weight of around 107.868. Chemically speaking, sliver is not very active. The metal cannot be dissolved in dilute alkaline and acids, it is however soluble in concentrated sulfuric and or nitric acid.
The metal also does no react with water or oxygen at ordinary temperatures. Sulfides and sulfur can tarnish silver through the formation of sulfur silver sulfide (a モバイル カジノ product of a combination reaction between silver and sulfides or sulfur) on the surface of the metal. Eggs can quickly and easily tarnish silver as they contain a significant amount of sulfur as one of the constituents of protein. Small amounts of sulfide (that occurs normally in the atmosphere) as hydrogen sulfide can be added to natural gas and be used in damaging the silver.
The back silver sulfide is amongst the salts that are most insoluble in aqueous solution. This is a property that o good use for separating silver ions from many other positive ions. Due to its high level of ability to be beaten into a sheet or drawn into a thin wire, silver is most often used in making jewelry or ornamental furniture.

